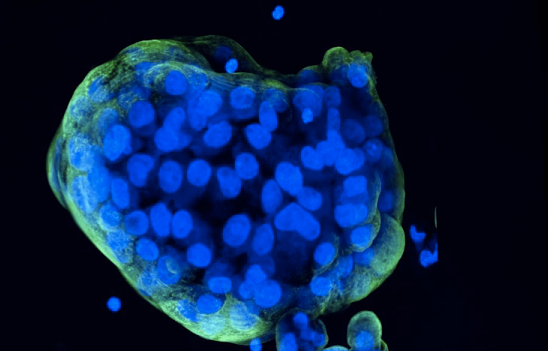
A novel method to bioprint miniature tumor organoids was developed by scientists from the UCLA Jonsson Comprehensive Cancer Center. The method will allow researchers to study and analyze individual organoids in great detail, which could help identify personalized treatments for individuals with rare or hard-to-treat cancers.
The research team reported on their developments in Nature Communications.
High-throughput drug screening is an established approach to investigate tumor biology and identify therapeutic leads, the authors wrote. Traditional platforms use two dimensional cultures, but these do not accurately reflect the biology of human tumors, the authors noted. In contrast, “three-dimensional (3D) tumor organoids are promising models for precision medicine that can be established rapidly and effectively from a variety of cell lines and tissue sources, and accurately mimic a patient’s response to therapy,” the team noted.
These miniaturized tumors, called organoids, can be grown in a lab using cell lines or patients’ own cells to better understand human biology and diseases. By recreating patient tumors, researchers can test different drugs to see if the tumor will respond well or poorly to the treatment. This can make it easier for physicians to choose the best therapy for their patients.
“Tumor organoids have become fundamental tools to investigate tumor biology and highlight drug sensitivities of individual patients,” explained Soragni. “However, we still need better ways to anticipate if resistance could be arising in a small population of cells, which we may not detect using conventional screening approaches. This is truly important, particularly as organoid-based drug predictions are starting to be leveraged clinically.”
While these mini tumors have helped improve drug modeling and are becoming invaluable tools for testing the efficacy and safety of potential drugs, it is still challenging for current models to capture underlying tumor heterogeneity which often drives clinically observed resistance to therapy. “ … even these more clinically relevant model systems such as three-dimensional tumor organoids can be difficult to scale and screen,” the authors pointed out. One of the main limitations of this approach is that current methods fail to capture changes or differences within the organoid samples that may be responsible for the resistance to therapy that is observed in clinical settings.
To overcome these challenges, the team of researchers created a method that uses a bioprinting technique to print cells in a thin layer of support extracellular proteins to give rise to 3D mini-tumors without altering the tissue histology and gene expressions. “Bioprinting, a technique for precise, reproducible deposition of cells in bioinks onto solid supports, is rapidly gaining traction in cancer biology, Soragni and colleagues noted.
The team combined their bioprinted cells with high-speed live cell interferometry (HSLCI), an imaging system that offers a non-destructive approach used to observe and measure the weight of living cells in real time. “HSLCI allows non-invasive, label-free tracking of various features of bioprinted organoids over time, including size, motility, and mass density at single-organoid resolution,” the investigators explained. These methods were then combined with machine learning algorithms to analyze and measure individual organoids.
“By using this method, we are able to accurately measure the masses of thousands of organoids simultaneously,” explained Michael Teitell, MD, PhD, director of the UCLA Jonsson Comprehensive Cancer Center and co-senior author of the study. “This information helps identify which organoids are sensitive or resistant to specific therapies, which can be used to quickly select the most effective treatment options for patients.”
With the new method combination, researchers confirmed that they could measure the growth patterns of the bioprinted tumor cells over time to see how the cells responded to different drugs or treatments. “Using cell lines as a model for 3D tumor cell growth, we demonstrate that bioprinted cells deposited in uniform, flat layers of extracellular matrix allow label-free, time-resolved, non-destructive quantification of growth patterns and drug responses at single organoid resolution,” they noted. Teitell further noted, “The measurements were done in a way that did not damage or destroy the organoids, allowing for non-invasive analysis of their growth and drug responses.”
The researchers were able to identify an effect of certain drugs on cells as soon as six hours from adding the therapies. The team also identified small groups of cells that did not respond to the drugs, even within very homogeneous cell line samples consisting mostly of cells that were responsive to the treatment.
“Key limitations towards the broad adoption of functional precision medicine have been the creation of physiological culture models, the development of high throughput systems, and the difficulty in measuring organoid heterogeneity,” the investigators stated. “Our pipeline overcomes each of these barriers by incorporating a robust 3D organoid bioprinting protocol and an imaging approach that facilitates single-organoid analysis of response to treatment.” The researchers will leverage the new approach to uncover novel therapeutic avenues and mechanisms of resistance to eventually develop personalized treatment strategies. Soragni added, “This new pipeline has enhanced the quality and depth of information we can get from drug screening of 3D models of disease. We are now applying the same approach to organoids established from hard-to-treat, rare cancers.”
The novel method may lead to more informed clinical decision making when selecting a treatment approach.













